Last Updated on January 6, 2022 by
Table of Contents
Capillary Action
Capillary action can be defined as the ability of a liquid to flow through thin tubes, cylinder or permeable surfaces. This occurs due to the intermolecular forces (adhesive and cohesive) between the liquid and the surfaces. The diameter of the tube and the gravitational force determines the extent of liquid rise. The liquid will rise higher if the tube is narrower.
Forces in capillary action
The rise or fall of the fluid in capillary action is governed by the balance of the adhesive force and the cohesive force. The adhesive force and cohesive force are intermolecular forces
The three important forces that determine the capillary action are explained below.
Cohesive Force
Cohesive force is the intermolecular force that is responsible for the mutual attraction between the molecules and because of this the liquid resist separation. This is the force between the molecules of the same substance. For example, rain does not fall as a fine mist, it falls as drops. The cohesive force pulls the molecules together forming droplets.
Adhesive Force
Adhesive force is the force of attraction between the molecules of unlike substances. The substance sticking together and the forces between unlike charges are examples of adhesive forces.
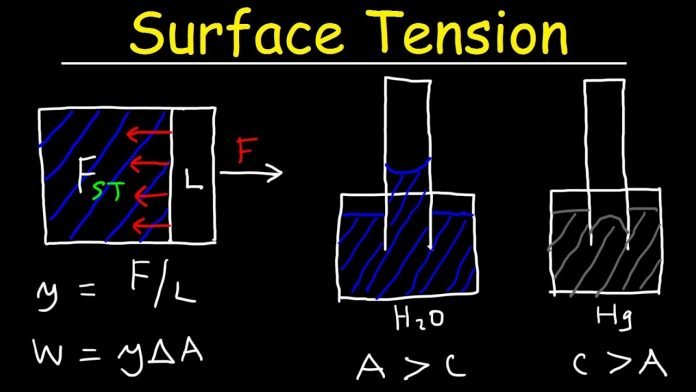
Surface Tension
The phenomenon of surface tension is caused due to the cohesive force between the molecules of the liquid. The molecules on the surface of the liquid are attracted only to the surface molecules and the molecules inside the liquid, whereas the interior molecules are attracted to all the molecules surrounding it. Therefore the energy state of the interior molecules is lower than the molecules of the exterior. The molecules of the liquid try to maintain a minimum surface area, thus allowing a maximum number of molecules to have a lower energy state. This forms a surface film and can cause the things denser than water to float on the surface.
Comparison of Capillary Action in Water and Mercury
In water, the strength of the adhesive force is larger than the cohesive force. The water forms concave meniscus and the surface of the water inside the tube is higher than the surface of water outside the tube. This is called capillary attraction.
In mercury, the cohesive force is stronger than the adhesive force. The meniscus formed by mercury in the tube is convex and the surface of mercury inside the tube is lower than the surface of mercury outside the tube. This is called capillary repulsion.
Application of capillary action
- The oil rises up the wick of the lamp due to capillary action.
- The towels absorb the water due to the capillary action of the cotton present in the towel.
- The plants cannot survive without capillary action. The water from the soil rises up due to capillary action.
- A technique called chromatography uses capillary action. In this technique, a layer of water is used to separate mixtures from substances.
Dimensions of Surface Tension
The force per unit length perpendicular to the line drawn to the surface is called surface tension. The SI unit of surface tension is Newton/metre.The dimensional formula or dimensions of surface tension is given by,
Dimensional formula for Surface Tension = M1 L0 T-2
Where,
M is the Mass
L is the Length
T is the Time
Since surface tension is the force acting per unit length. Substituting the dimensional formula for force and length gives the dimensional formula for surface tension. Therefore, surface tension is dimensionally written as M1 T-2.