Last Updated on March 12, 2024 by admin
The journey of medical device creation is a complex one, filled with myriad requirements and regulations to ensure safety and efficacy. To make a meaningful impact, these devices need not only be innovative but must also adhere to strict regulatory compliance. While these regulations might be seen as a stumbling block by some, they are in fact, an essential part of product innovation, driving manufacturers to find better, safer, and more effective solutions.
When we dive deeper into the world of medical devices, one standard that stands out is ISO 13485, an internationally recognized quality management system specific to the medical device industry. It serves as a solid foundation upon which manufacturers can build and demonstrate their commitment to regulatory compliance. Navigating the regulatory pathway for medical devices can provide valuable directions on how to excel in aligning with this standard, thus bolstering product dependability and safety.
The Double-Edged Sword of Compliance
Regulatory compliance in the medical device industry can be a double-edged sword. On one hand, the strict regulations can often be perceived as hurdles to rapid innovation. They necessitate rigorous testing and validation, extensive documentation, and a high degree of traceability. This can add time, cost, and complexity to the device development process.
On the other hand, these compliance measures ensure that medical device innovation does not occur at the expense of patient safety. They push companies to demonstrate that their device is not only novel but also safe and effective for its intended use. Moreover, achieving compliance can bolster the credibility of the company and its products, potentially leading to increased market adoption.
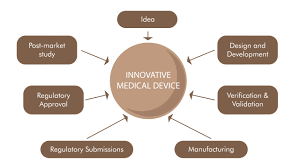
Regulatory Compliance as a Driver of Innovation
Rather than stifling innovation, regulatory compliance often propels it. It drives manufacturers to seek innovative methods that not only adhere to regulations but also improve device efficiency, affordability, and patient outcomes.
For instance, to meet compliance requirements, many manufacturers have begun to incorporate advanced technologies, like Artificial Intelligence (AI), and Machine Learning (ML) into their devices. Such technologies can enhance device performance, improve predictive maintenance, and optimize patient care – all while ensuring adherence to regulatory standards.
Additionally, regulations also promote innovation in process management. ISO 13485, in particular, emphasizes risk management throughout the device life cycle. This emphasis pushes companies to develop novel risk assessment methodologies and strategies, fueling process innovation.
ISO 13485: A Path to Compliance and Beyond
ISO 13485 is a powerful tool for medical device companies seeking to ensure regulatory compliance and spur innovation. This standard, dedicated to quality management systems, focuses on process consistency, risk management, and continuous improvement – key elements in driving both compliance and innovation.
Understanding and implementing this standard effectively can be a complex task. A comprehensive iso 13485 implementation guide can provide a roadmap for device manufacturers to achieve and maintain this certification, covering aspects like quality management, regulatory requirements, risk management, and customer satisfaction.
The Way Forward
In conclusion, while it may seem that regulatory compliance and innovation stand on opposite sides of the spectrum, they are in fact, deeply interwoven. Regulations like ISO 13485 not only ensure the safety and effectiveness of medical devices but also promote innovation in product design and process management.
For medical device manufacturers, embracing these regulations as an integral part of their innovation strategy can lead to the development of devices that are not only novel and effective but also trusted by the medical community and patients alike. As we move forward, it is clear that regulatory compliance will continue to shape the landscape of medical device innovation in remarkable ways.
Apart from this, if you are interested to know more about Essential Equipment for Starting a Medical Clinic then visit our Health category.






















